The immune system is that part of our body which tells us who is “us,” and who is “other.” The immune system has to recognize all the cells and tissues in the body, know which ones are friendly, and which ones are foreign invaders and worthy of a vigorous attack.
In autoimmune diseases - immune system dysfunction - the body gets confused. In chronic fatigue/fibromyalgia, the immune system is weak and can’t mount much of an attack against invaders. In asthma, the immune system may be too challenged to communicate properly, and simply stays “on” all the time.
The statistics keepers tell us one in 12 Americans will develop an autoimmune disorder. Compare that to the estimate that one in 20 adult Americans will have heart disease.[1] Yes, autoimmune diseases are more common than heart disease, the number one killer. Clearly, many people are finding that their immune system is failing them.
Fibromyalgia, chronic fatigue syndrome, multiple chemical sensitivity, systemic lupus, multiple sclerosis, and Gulf War Syndrome all are relatively new and all appear to be autoimmune in nature.
The human body swarms with “good” bacteria that digest our food and serve our organs. The number of bacteria - foreign cells - living within the body of the average healthy adult human is estimated to outnumber human cells 10 to 1.[2] So clearly, the immune system must know how to distinguish “good” foreign cells from “bad” foreign cells worthy of an attack.
 The immune system may act like immigration control or homeland security, regulating the type and number of our microbial menagerie, working “to maintain communities of bacteria in balance.”[3] This theory was proposed by Margaret McFall-Ngai, a University of Wisconsin biology and immunology professor. This hypothesis could have wide-ranging consequences for medicine because a growing number of health problems, from inflammatory-bowel disease to obesity, have been linked to bacterial communities out of balance, rather than to a lone pathogen wreaking havoc.
The immune system may act like immigration control or homeland security, regulating the type and number of our microbial menagerie, working “to maintain communities of bacteria in balance.”[3] This theory was proposed by Margaret McFall-Ngai, a University of Wisconsin biology and immunology professor. This hypothesis could have wide-ranging consequences for medicine because a growing number of health problems, from inflammatory-bowel disease to obesity, have been linked to bacterial communities out of balance, rather than to a lone pathogen wreaking havoc.
Louis Pasteur, who created the “germ theory” of disease, is said to have refuted his own theory toward the end of his life. Speaking of the body as the “terrain” in which the germs live, he supposedly told his good friend Professor Louis Renon, “The pathogen is nothing. The terrain is everything.”[4,5,6] This is a reference to the fact that “bugs,” and other things that make us ill show up when the inner terrain is weak in much the same way flies come to stagnant water. It explains why one person succumbs to the flu and the next doesn’t. Whether Pasteur did recant his germ theory or not, it definitely ended up enshrined in the medical books, and a system of medicine developed that “fights” germs instead of focusing on the creation of a robust immune system.
Today, the mindset is changing.
For example, Daniel Frank at the University of Colorado, Boulder, is part of a team exploring the microbial family’s role in inflammatory bowel diseases in the gut and how “the family” changes in relation to disease.
And Ruth Ley at Washington University in St. Louis is part of a team that several years ago discovered obesity was associated with changes in the relative abundance of certain types of bacteria in the digestive tract.
Michael Price, writing in Science Magazine in 2010, said:
“All those Lucky Charms and Big Macs that people in the developed world scarf down could explain why they are more susceptible to allergies, autoimmune disorders, and inflammatory bowel disease than are residents of agrarian societies. New research suggests that people living in rural Africa have a healthier mix of microbes in their guts than do their Western counterparts, which may protect them from the intestinal diseases that are common in modern developed countries … Modern sanitation and medicines have further changed the types of bacteria people encounter.”[7]
It now appears that just as people have a particular blood type, they also have a particular "bug" type. In 2011, scientists studied people from industrial nations and found their human gut ecosystems fall into one of three distinct types. Like blood groups, these gut types are independent of traits like age, gender, nationality and body-mass index.[7a] “We found that the combination of microbes in the human intestine isn’t random,” said lead researcher Peer Bork.[7b] The discovery of the blood types A, B, AB and O had a major effect on how doctors practice medicine. This new discovery of enterotypes will likely the change the practice of medicine. Bork speculated doctors might be able to use enterotypes to find alternatives to antibiotics, for example. Instead of trying to wipe out disease-causing bacteria that have disrupted the ecological balance of the gut, doctors could try to provide reinforcements for the good bacteria.
McFall Ngai says it is quite possible that mapping and understanding the human microbiome may be as important or more important to understanding human health than mapping and understanding the human genome.
She says we are also going to have to change our idea of “good” and “bad” bacteria. Aside from a few “professional pathogens” like the bubonic plague-causing Yersinia pestis, most bacteria are not inherently good or bad, McFall-Ngai says. Instead, bacterial effects are highly context-dependent: She reported in 2004 that a common bacterial “toxin” which causes tissue damage under some circumstances, also plays a critical role in host tissue development. “We have thousands of bacteria that live with us, and yet there are only around 100 bacterial pathogens,” she says. Given the numbers, “it seems like these pathways and these molecules are likely to be ‘symbiosis’ pathways more than ‘anti-pathogen.’ The language is ancient and highly conserved.”
IMMUNE SYSTEM JOB #2 - Regulating the bugs & marshalling the troops
The first line of defense against “bad” foreign cells is called innate cellular immunity (also known as a Th1 immune process). Our bodies respond to foreign invaders (e.g. bacteria) by stimulating the production of cells (the ground troops) that normally surround or engulf foreign antigens and destroy them.
The Th1 system depends on “dendritic” cells, which are called by different names in different organ systems:
 • Blood = macrophages, monocytes, or granulocytes
• Blood = macrophages, monocytes, or granulocytes
• Tissues = fibroblasts, endothelial cells, and Kupfer cells
• Brain = glial cells
• Spleen = sinusoidal cells
These are the “recyclers” of the body. They will engulf anything foreign to the body - chemicals, large food molecules, heavy metals, toxins, bacteria, viruses… Their function is to identify and destroy any foreign invader.
Only when these cells are overwhelmed do we begin to use the more specific humoral immune system, (the Th2 immune process). This Th2 process is a complex series of events triggered after antigens enter the body. Part of the reason that vaccinations can be so challenging for some of us is the fact that they bypass the body’s cellular immune system. We inject the antigen directly into the muscle tissue, bypassing the skin and mucus membranes which would ordinarily be the first responders.
The vitamin D receptor on the cell membrane regulates the number of immune cells put into circulation. The number of immune cells is vital to the response of the immune system to invaders. The receptor is regulated by various forms of vitamin D.
• 1,25 di-hydroxy Vitamin D (1,25 D) is the form which turns the receptor on, thereby increasing the number of macrophages released. Vitamin D receptor activation causes an increase in the synthesis of antimicrobial peptides which can kill bacteria, viruses and other invaders immediately, in an “innate” or “cellular” immune response.
• 25-OH Vitamin D (25 D), the form that we commonly take as a supplement or eat in our food, is the form which turns the Vitamin D receptor off, thereby decreasing the number of macrophages released. It often works well, temporarily, if the condition is one of high inflammation. However, 25 D is converted to the 1,25 D form by the kidneys, and by sunlight acting on the 25 D form in the skin, which then turns the receptor back on, thus potentially resulting in increased inflammation in the final analysis.
So, is it bad to take a vitamin D supplement? Of course not - if you don’t have sarcoidosis or other Th1 autoimmune inflammatory disease. Many of us are indeed vitamin D deficient, our immune systems are weak, we do not produce the macrophages that we need to ward off illness. Our bodies are perfectly capable of making the homeostasic adjustment between 25 D and 1,25 D so that our immune systems do not become hyperactive. However, some of us are not able to make that adjustment - our Th1 systems are already so far out of kilter that taking more vitamin D supplement just makes things worse. For these people, vitamin D balance is much more complicated than just taking a pill.
IMMUNE SYSTEM JOB #3 - Attacking the Invaders
Is inflammation a good thing? Or a bad thing? Well… Inflammation is a sign that something foreign has invaded the body. But how much of a response is it worth?
For example, toxins (chemicals, bacteria, pollens, etc) stimulate the production of macrophages (inflammation) to take away the unhealthy stuff we breathe into our lungs. We cough a little, produce a little mucus, trap the toxin in our mucus, cough a little more and cough it out.
But what happens if the inflammation then does not subside? What if we forget to furlough the troops? We continue to produce mucus, plug up our airways, continue to cough, begin to wheeze because the airways are narrowed, and now we are diagnosed with a disease called asthma. Now we have to use an inhaler to open the airways, or a steroid to decrease the inflammation. It’s a delicate balance. Insurance will pay for the treatment of asthma, where it may not pay for discovering and treating the cause of the inflammation in the first place. Another delicate balance… this time between our health and our wallet.
Finding the cause of inflammation can be tricky. An abscess is pretty clear evidence of bacterial infection. So is cellulitis (infection of the soft tissues of the skin). So is pneumonia, otitis media, vaginitis, sinusitis. A bruise is often followed by a bit of inflammation. However, an injury needs a different response than a foreign invader. How does the body distinguish between a bruise and an infection?
A little biology lesson is in order.
Each of your cells has a marker as distinctive as a fingerprint. Your immune system can see markers on the outside of every one of your cells and distinguish them from other cells which have different markers. When a different fingerprint shows up, those cells are recognized as “foreign,” and targeted for destruction.
When foreign cells appear, your immune system will send out an army to attack them. This response can be delayed, because it requires preparation, akin to sending an army to a foreign country - it takes a few hours to a few days for the team to get itself organized and start producing specific immunoglobulins.[8] That is why when you catch the flu virus, you get symptoms for several days before your immune systems starts to kick in and you begin to feel better.
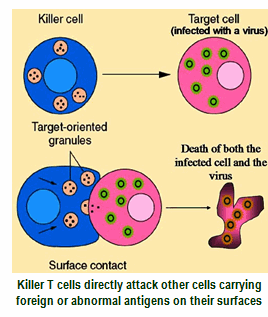 Just as an army has ground troops, pilots, and sailors, your immune system has different types of immune cells with different defensive functions:
Just as an army has ground troops, pilots, and sailors, your immune system has different types of immune cells with different defensive functions:
• Macrophages and neutrophils = these are the ground troops, cells that respond to all invaders and toxins by creating an inflammatory state, releasing cytokines and interleukins which promote further inflammation
• Eosinophils and mast cells = these are the assault troops, respond to parasitic invasions and allergies
• Natural Killer Cells = these are the gunners, destroying cancer cells and other tissues which do not have “your fingerprint”
• Specific lymphocytes (T-helper, T-killer, B cells) = these are the Special Forces, they already have experience with the invaders or toxins and are up to speed, ready to attack as soon as the invader returns.
The body has many choices when it comes to foreign invaders. It will try to use the simplest method first, before moving on to the next level.
JOB #4 - Pep talks: Why would the troops take a vacation, or even mutiny?
Clearly with so much autoimmune disease in the population (1 adult in 12), the human immune system is going haywire pretty frequently. Why? Lots of reasons; here are our top 12:
1. Chronic low thyroid - The thyroid gland plays a crucial role in maintaining the body’s defenses. But a genetic defect in the human population has increased, thanks to the discovery of penicillin. The defect? Some people are chronically hypothyroid because the thyroid in their bloodstream doesn’t get inside the cells where it is needed.[9] Before the advent of penicillin, those who were low on thyroid were more susceptible to infections and often died before reaching the childbearing years. With the advent of antibiotics, those people lived, and passed on the defect. Individuals with hypothyroidism are definitely more susceptible to viral and bacterial infections, and their immune systems have more opportunity to become confused.
2. Chronic low level infections - We see mutated forms of some bacteria and viral strains that require stronger immune systems than ever before. MRSA and Lyme are just two such examples. A 2007 study of immune cells battling a chronic viral infection shows that T-cells become exhausted and depleted in their struggle against a virus.[10]
3. Evolution – A Rice University study suggests the immune system has evolved a near-perfect balance for producing antibodies that are both effective against pathogens and unlikely to cause autoimmune disease.[11] The system could in fact have evolved to produce better antibodies in less time. However, doing so would make the immune system far more likely to attack its own body’s tissues.
4. Stress – Chronic psychological stress raises cortisol levels and turns off the inflammatory response to infection.[12] Conversely, having a positive attitude seems to correlate with an increased ability of the immune system to fight diseases.
5. Sugar - Today’s highly processed food favors bacteria that thrive on refined sugar. Research has shown that obese people carry different bacterial communities in their guts than lean people.
6. Fats – For years we were told to eat butter substitutes full of unhealthy trans fats and to avoid “saturated fats” like the plague. Ditch the coconut oil too, they said. WRONG! Researchers found that a loss of saturated fatty acids in the lymphocytes was responsible for declines in white blood cell function.[13] They found they could correct cellular deficiencies with myristic acid found in coconut oil and dairy fats. Myristic acid is a very important fatty acid used by the body to stabilize proteins used in the immune system and to fight tumors.
7. Environmental assaults - Autoimmune diseases have increased dramatically in recent decades, in parallel with our use of chemicals like pesticides, dioxins, heavy metals, and plastics. Many think the two are related. Scientist Rodney R. Dietert studied the effects of exposure to biological materials, drugs, medical devices, chemicals, and other environmental factors on the developing immune system in fetuses, infants and children. His research suggests links between environmental factors and an increased risk of asthma, autism, diabetes, leukemia, and other diseases.[14]
8. Vitamin A deficiency - Lack of vitamin A is a precondition for the development of many if not all autoimmune diseases. Exposure to these environmental chemicals poisons the process of making and transporting vitamin A and vitamin A hormone in our bodies.[15]
9. Vitamin/Hormone D deficiency - D plays a role in regulation of both the “infectious” immune system and the “inflammatory” immune system.[16] A deficiency of some forms of Vitamin D is associated with several autoimmune diseases including multiple sclerosis, Sjogren’s Syndrome, rheumatoid arthritis, thyroiditis, and Crohn’s disease.[17]
10. Thick blood - Hypercoagulability is increasingly common. When blood gets thick, it doesn’t move as well through tiny capillaries. Cells become starved for oxygen and nutrients. Pain can result, as in fibromyalgia. Many chronic conditions such as attention deficit disorder, autism and schizophrenia, as well as multiple sclerosis, Parkinson’s disease, irritable bowel, sleep disorders and more are related to elevated blood viscosity and organ dysfunction caused by impeded, insufficient blood flow.
11. Biofilm - Microbes hide themselves from the immune system by making a protein shield called biofilm. The immune system has no way of seeing the organisms behind this biofilm - a common feature of many chronic inflammatory diseases.
12. Estrogen - in lower amounts estrogen does appear to be stimulatory to the immune system. Higher levels, as occur during pregnancy or with hormone replacement therapy, appear to modulate the Th1 system and may well decrease Th1-mediated immune responses.
JOB #5 - Friendly Fire: What went wrong? Why are my own troops attacking me?
 How does the horrific accident of “friendly fire” occur? Why would one part of our body decide to attack and destroy another part of our body? And why is there a battle in the first place? Why do we not simply live in harmony with all these microbes?
How does the horrific accident of “friendly fire” occur? Why would one part of our body decide to attack and destroy another part of our body? And why is there a battle in the first place? Why do we not simply live in harmony with all these microbes?
Many systemic illnesses are associated with immune system dysfunction, from allergies to diabetes and other auto-immune diseases, to chronic fatigue syndrome[18] and heavy metal toxicity.[19,20]
Auto-immune diseases occur when for some reason, the body believes that its own tissues are foreign - brain tissue in autism or multiple sclerosis, thyroid tissue in autoimmune thyroiditis, pancreas in diabetes, muscle tissue in myositis, joint tissue in rheumatoid arthritis. Because the body sees these tissues as foreign, it mounts an immune response, resulting in inflammation and tissue destruction.
Chronic fatigue syndrome and fibromyalgia can be the end result of stress, inflammation, viral infection, or may simply appear for no apparent reason. Both these syndromes are associated with immune system dysfunction, and both have a tendency to appear after a significant viral illness which simply never seems to heal completely.
Heavy metals are toxic metallic elements like lead, arsenic, cadmium, mercury and others which have been linked to neurobehavioral abnormalities (lead), chronic kidney damage (cadmium), GI dysfunction (arsenic) and sensory and neurologic impairments (mercury). The fetus is especially susceptible, because of the significantly neurotoxic effect of these metals on the developing brain. Heavy metals are known to induce autoimmunity in mice.
Bacteria can also confuse the body into thinking that its own tissues belong to somebody else. Ordinarily, bacteria are clearly identified by their own individual fingerprint. However, under certain circumstances, all bacteria can begin to produce a protein called “biofilm” which acts like a shield. When the bacteria hide behind this shield, the cells of the immune system are no longer able to identify the bacteria as “foreign” because they don’t see them. All the immune system sees is its own cells which are too big to destroy. When our own body’s cells become abnormal – as in cancer cells – the rest of the immune system rallies around and attempts to destroy the abnormal cells – brain cells, joint cells, nerve cells, intestinal lining cells…
A little more biology…
In the case of the autoimmune disease – sarcoidosis for example – the sarcoid granulomas appear to convert the dietary form of Vitamin D, called 25-D to the active hormonal form, called 1,25 D, producing large amounts of 1,25 D, far in excess of the body’s requirements.
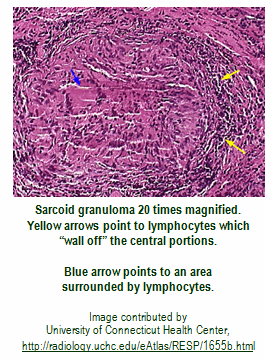 As the circulating levels of 1,25 D increase, more pro-inflammatory macrophages are made. That triggers more ACE (angiotensin converting enzyme) stimulating the release of a cascade of Th1 pro-inflammatory factors which then stimulate the differentiation of yet more macrophages - thus creating a vicious cycle of inflammation. Normally this cycle is self-limiting, because the levels of 1,25D and 25 D are supposed to be in balance. However, with sarcoidosis patients, and with many other patients suffering from autoimmune inflammatory diseases, 1,25 D is synthesized within the granulomas in the lungs. Therefore, no matter what the original cause of the excessive inflammation, if the result is granulomas, the patients will have a self-perpetuating disease process through elevated levels of 1,25 D.
As the circulating levels of 1,25 D increase, more pro-inflammatory macrophages are made. That triggers more ACE (angiotensin converting enzyme) stimulating the release of a cascade of Th1 pro-inflammatory factors which then stimulate the differentiation of yet more macrophages - thus creating a vicious cycle of inflammation. Normally this cycle is self-limiting, because the levels of 1,25D and 25 D are supposed to be in balance. However, with sarcoidosis patients, and with many other patients suffering from autoimmune inflammatory diseases, 1,25 D is synthesized within the granulomas in the lungs. Therefore, no matter what the original cause of the excessive inflammation, if the result is granulomas, the patients will have a self-perpetuating disease process through elevated levels of 1,25 D.
When we measure vitamin D levels, we generally measure 25 D and not 1,25 D because the latter is a more expensive test. If we see low 25 D levels, we think that the body does not have sufficient vitamin D. We may even supplement vitamin D. And it may help for a while, because 25 D turns off the vitamin D receptor, reducing the number of inflammatory macrophages. However, the system will continue to produce 1,25 D spontaneously (and in response to the higher 25 D levels), thus re-stimulating the inflammatory process which we were attempting to treat. If the level of 1,25-dihydroxy vitamin D in the blood is elevated (above 38-45pg/ml), or the 25-hydroxy vitamin-D depressed (below 20 ng/ml) then it is pretty certain that this process is in play.
Sarcoidosis restricts lung function, negatively impacts lymph nodes and other organs, and usually becomes end-stage within one or two decades of diagnosis.
There is another inflammatory process at work
What causes the Th1 system to be turned on excessively in the first place? Sarcoidosis patients - and others with chronic inflammation - are found to have large numbers of cell wall deficient bacteria (CWD) living within their macrophages. In other words, the immune system has identified these bacteria as something foreign. Macrophages have engulfed the bacteria, but appear unable to destroy them.
Almost every micro-organism can, under the right circumstances, turn into a CWD form. This ability is called pleomorphism - and its purpose appears to be avoidance of attack by our immune system. This is clearly seen with mycoplasma organisms which cause pneumonia, and spirochetes which cause syphilis and Lyme disease, yet it has also been demonstrated with most other bacteria. In other words, most bacteria are capable of morphing into a CWD form which is not recognized by our immune systems.
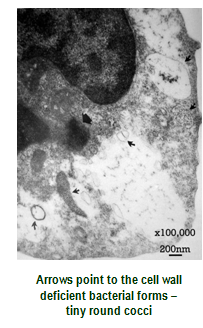 CWD organisms are very tiny - many of them can fit into a macrophage and make their own biofilm to hide behind. Biofilm is like the stuff that accumulates on your teeth (where it is called plaque), in your blood vessels (called arteriosclerosis), in wounds (called necrotic tissue), etc. It is an impenetrable barrier behind which a multitude of organisms can hide. Biofilm allows CDW organisms to exist within the macrophage without any problem whatsoever. They are not destroyed, but they do provoke the macrophage into creating inflammatory substances called cytokines which have a major damaging impact on the rest of the body.
CWD organisms are very tiny - many of them can fit into a macrophage and make their own biofilm to hide behind. Biofilm is like the stuff that accumulates on your teeth (where it is called plaque), in your blood vessels (called arteriosclerosis), in wounds (called necrotic tissue), etc. It is an impenetrable barrier behind which a multitude of organisms can hide. Biofilm allows CDW organisms to exist within the macrophage without any problem whatsoever. They are not destroyed, but they do provoke the macrophage into creating inflammatory substances called cytokines which have a major damaging impact on the rest of the body.
Why can we not culture these bacterial invaders? Today’s laboratory tests are of little use until the immune system starts to recognize these intracellular invaders. Hundreds of these tiny bacteria can live inside a single cell. They are too small to be seen with conventional optical microscopes. The host cells live for a relatively long time. There is little cell death (apoptosis), so very few bacteria are released into the bloodstream, and they are hard to detect with lab equipment. Because our immune system does not recognize these bacterial invaders as foreign, since they are in the cell-wall-deficient form, no antibodies are created. The bacteria live within the cells in stable colonies surrounded by biofilm, so they cannot be attacked by the body’s normal defenses. Lab tests will not find antibodies to these bacteria.
IMMUNE SYSTEM JOB #6 – What if the troops need better ordinance?
Standard allopathic treatment for almost all autoimmune diseases involves decreasing inflammation, either by using steroids to decrease the inflammatory response, or methotrexate - a cancer drug - to stop the development and maturation of our own inflammatory cells. However, steroids do not cure, and can cause significant deterioration of health over the long term. Methotrexate causes problems with all rapidly dividing cells in the body - intestinal tract, mouth, skin, nails, hair.
The Marshall Protocol
The Marshall Protocol was developed in an effort to treat chronic diseases thought to be caused by cell wall deficient (CWD) bacteria. In 2008, Trevor Marshall, PhD co-chaired the 6th International Congress on Autoimmunity and presented eight years of data indicating that intraphagocytic bacteria are able to block the vitamin D receptor, leading to abnormally low measured vitamin D levels (25 D) and causing excessive Th1 immune response. This state of affairs can trigger persistent infection and inflammation and thus cause many chronic diseases, not just sarcoidosis.
“Dysregulation of vitamin D has been observed in many chronic diseases, including many thought to be autoimmune,” said J.C. Waterhouse, Ph.D., founder of the Chronic Illness Support and Research Association. “We have found that vitamin D supplementation, even at levels many consider desirable, interferes with recovery in these patients.”[21]
 The Marshall Protocol uses both an Angiotensin II blocker and minuscule doses of antibiotics infrequently given. The aim of this protocol is to decrease the inflammatory changes seen with many chronic diseases, including sarcoidosis and other autoimmune diseases, and then to stop the CDW bacteria from making their biofilm protein, so that our own innate immune systems can then first see the bacteria, and then kill them. The treatment appears to be effective also for other chronic inflammatory conditions with activation of the Th1 immune response, including some cases of chronic fatigue syndrome, fibromyalgia, chronic yeast infections, interstitial cystitis, rheumatoid arthritis and other difficult-to-treat chronic microbial infections.
The Marshall Protocol uses both an Angiotensin II blocker and minuscule doses of antibiotics infrequently given. The aim of this protocol is to decrease the inflammatory changes seen with many chronic diseases, including sarcoidosis and other autoimmune diseases, and then to stop the CDW bacteria from making their biofilm protein, so that our own innate immune systems can then first see the bacteria, and then kill them. The treatment appears to be effective also for other chronic inflammatory conditions with activation of the Th1 immune response, including some cases of chronic fatigue syndrome, fibromyalgia, chronic yeast infections, interstitial cystitis, rheumatoid arthritis and other difficult-to-treat chronic microbial infections.
When the vitamin D receptor is turned off, the body stops making antimicrobial peptides. When the receptor is turned on, it makes those peptides and is able to kill the CWD bacteria. If we accept current scientific literature stating that 1,25 D is actively produced within sarcoid granulomas[22], then the idea of reducing the levels of D3 to treat the imbalance which we know as sarcoidosis makes sense. We are simply reducing the activation of the vitamin D receptor to reduce the production of inflammatory cytokines, and then blocking the Angiotensin II receptor to further reduce the production of more inflammatory cytokines. Once the levels of inflammation are reduced, we give very small doses of bacteriostatic antibiotics which prevent the CWD bacteria from synthesizing the RNA that they need to produce their biofilm proteins, in effect starving the CWD bacteria. Once the bacteria are finally killed by our immune system’s antimicrobial peptides, the excessive inflammation goes away, and the patient is considered to be “in remission.”
Of course, in the meantime, we often see the so-called “Jarish-Herxheimer” reactions - also known as “Herx” - which temporarily increase symptoms because of death of bacteria, and the body’s inflammatory response to try to clean up the debris.
Trevor Marshall himself developed sarcoidosis in the 1970s and pursued research in biomedical engineering to understand the disease.
UNDERESTIMATING THE BUGS
 No matter how many antibiotics we threw at the pathogens, they continue to evade us and survive.
No matter how many antibiotics we threw at the pathogens, they continue to evade us and survive.
“One of the most foolish things humans did was to assume that bacteria were not intelligent,” says author and herbalist Stephen Buhner. Bacteria are living beings with a will to survive and an underappreciated ability to change and adapt.
Many have come to believe most of us have intracellular bacterial infections that never go away. They can be suppressed so there are no symptoms for a time, but the infections still lurk within us, waiting for an opportune moment to strike. One way these infections show up is as an auto-immune disease, so common today. The widespread use of antibiotics may be why we are seeing so many biofilms - they are the pathogen’s way to fight back at the antibiotics.
Chronic illnesses can involve multiple infections, different genetics, and multiple environmental toxins that impact them.
The whole process of therapy – and living, for that matter – is very much a balancing act. It is called homeostasis.
At the Arizona Center for Advanced Medicine, we look for the cause of the inflammation. We can perform a full medical work-up to identify possible infections, nutritional deficiencies, and contaminants like heavy metals. We look for food sensitivities, and make use of our FirstLine Therapy program to address problems of the Standard American Diet. We also reduce the burden of inflammatory heavy metals with chelation. We look for the best way to restore the body’s homeostatic balance, so that you can get on with your life, in health.
Call us, at 480-240-2600, or send an e-mail to info@ArizonaAdvancedMedicine.com, for a free 15-minute telephone consultation, to learn how we can help you.
